Food Labeling and Allergens
go.ncsu.edu/readext?1007630
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲Food Label Review
The Food & Drug Administration has published a Guidance document that will help provide answers to the most-frequently asked questions about creating a food label.
At minimum, food labels for packaged foods must include:
1. Product name/Statement of identity
2. Net contents listed in both the US Customary System (ounces, pounds) and metric (grams, kilograms, liters) or in units (4 Rolls)
a. Net quantity statement is placed in the bottom 30% of the principle display panel (PDP).
b. Exclude qualifying phrases such as: 4 large ounces or 5 juicy apples
3. Manufacturer or distributor’s name & physical address
4. Complete list of ingredients, in descending order of predominance by weight, including the common name of any allergens.
Other Food Label Requirements
- Use a print that is prominent and easy to read with letters that are at least 1/16 inch in height, based on the lower case ‘o’.
- An additional information panel should be located immediately to the right of the PDP (as the customer faces the product).
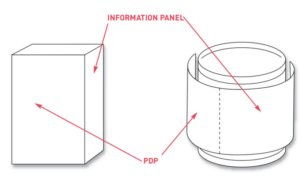
Image source: FDA. (2018, September 16). Guidance for industry: Food Labeling guide. U.S. Food And Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-food-labeling-guide
- Intervening materials may NOT be placed between required labeling information.
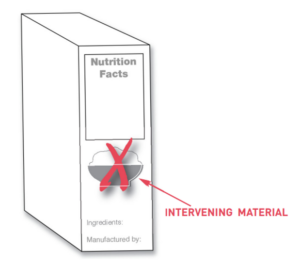
Image source: FDA. (2018, September 16). Guidance for industry: Food Labeling guide. U.S. Food And Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-food-labeling-guide
Nutrition Facts Panels
Nutritional labeling is required for most packaged foods with exceptions for*:
| Small businesses | Restaurants | Foods with no significant nutritional value |
| Bulk foods for further processing | Foods labeled “This unit not labeled for retail sale” sold in a multi-unit package | Fresh produce |
| Fresh seafood | Fresh meats | Donated foods |
For the sake of nutrition facts panels, the FDA defines a small business in one of two ways:
1 . Less than $500,000 annual gross sales
– OR –
2. Less than 100 full-time equivalent employees and less than 100,000 units sold annually
If you need assistance creating Nutrition Facts panels, ingredient lists or other labeling requirements, contact the EI4F Program a NC State Extension Program.
Food Allergens
Allergen presence and allergen mislabeling is currently the #1 reason for recalled product in the United States. For those who have food allergies, avoidance is the only option to avoid an allergic reaction, so food manufacturers are required to clearly communicate the allergen profile of their food products.
Any of the 9 identified allergens in food products must be included on the ingredient list on the packaging. There are no exemptions for the labeling of these 9 major food allergens.

9 Major Allergens for the United States |
||
| Milk | Eggs | Fish* |
| Sesame | Soy | Crustacean Shellfish* |
| Wheat | Peanuts | Tree Nuts* |
| * Allergen must be speciated (e.g. listed as ‘almonds’ not just ‘tree nuts’) | ||
Listing Allergens
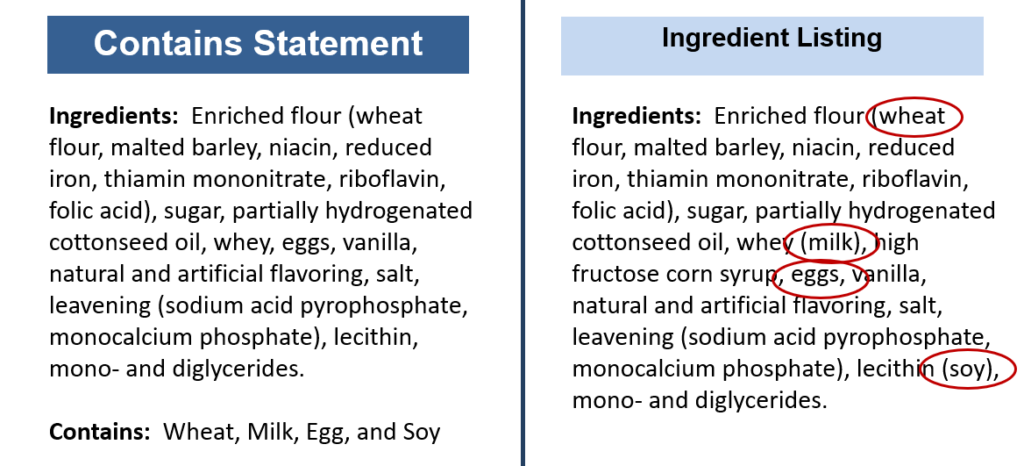
Allergens can be listed in one of two ways on your ingredient list.
- In a ‘Contains:’ statement. This statement must be located immediately under the ingredient list, with no intervening information, AND allergens must be listed by their common name.
- Identified by the allergen’s common name in the ingredient list. The common name of the allergen must be listed in parentheses directly after the ingredient that contains the allergen.
Precautionary Allergen Labeling
The FDA does not require precautionary labeling on food items. This is a voluntary statement that food manufacturers can put on their products that communicate the facility’s allergen profile to the consumer. They can include any of the following examples:
- May contain…
- Processed in a facility that also processes…
- Processed on shared equipment…
Be Aware of ‘Hidden’ Allergenic Ingredients
Some ingredients that food manufacturers use may contain allergens that are not clearly identified as allergens. This is especially important to be aware of in your raw materials and any changes to the allergen profiles of your ingredients. Below are a few examples of how allergens may be present under uncommon names for eggs, milk and soy.
| Terms that may indicate the presence of EGGS: | |||
| Albumin | Ovalbumin | Ovamucin | Ovamucoid |
| Lecithin | Ovovitellin | Livetin | Vitellin |
| Terms that may indicate the presence of MILK: | |||
| Lactose | Butter & butterfat | Casein | Rennet casein |
| Curds | Lactalbumin phosphate | Caramel color & flavoring | Artificial butter flavoring |
| Terms that may indicate the presence of SOY: | |||
| Gum arabic | Guar gum | Emulsifier | Hydrolyzed vegetable protein (HVP) |
| Miso | Vegetable gum | Carob | Textured vegetable protein (TVP) |
Source: Steinman, H. A. “Hidden” allergens in foods. The Journal of Allergy and Clinical Immunology, 98(2), 241–250.
Other Labels & Designations Seen on Food Packaging
Organic
The USDA oversees the ‘Organic’ certification in the United States. This is a voluntary program that companies can choose to partake in when they wish to communicate this quality about their products. There are 4 different organic label types:
- “100% organic” All ingredients are organic other than salt and water
- “Organic” A minimum of 95% organic ingredients
- “Made with organic ___” A minimum of 70% organically produced ingredients (can’t use the seal)
- Specific organic ingredient listings – Specific ingredient is identified as organic for products with less than 70% organic ingredients (can’t use the seal)

Date Labels
Date labels are applied at a company’s discretion, with the exception of infant formula which must bear a ‘Use by’ date. Date labels are to inform the customer of the date in which products have the highest quality and flavor and isn’t necessarily for food safety.
“Best if Used By” is the ideal terminology to convey quality standards. It indicates when a product will be of best flavor or quality. It is not a purchase or safety date.
“Sell By“ tells the store how long to display the product for sale for inventory management. It is not a safety date.
“Use By” is the last date recommended to use the product while at peak quality. It is not a safety date except for when used on infant formula.
“Freeze By” indicates when a product should be frozen to maintain peak quality. It is not a purchase or safety date.
Contact our Speakers:
| Lynette Johnston | Assistant Professor and Food Safety Extension Specialist | lynette_johnston@ncsu.edu | Food Safety Processors |
| Kate Nicholas | Food Safety Extension Associate | katenicholas@ncsu.edu | Food Safety Repository |
Webinar hosted on May 31, 2024
![]() “This work was supported by the intramural research program of the U.S. Department of Agriculture, National Institute of Food and Agriculture, Food Safety Outreach Program 1030908. The findings and conclusions in this preliminary publication have not been formally disseminated by the US Department of Agriculture and should not be construed to represent any agency determination or policy.”
“This work was supported by the intramural research program of the U.S. Department of Agriculture, National Institute of Food and Agriculture, Food Safety Outreach Program 1030908. The findings and conclusions in this preliminary publication have not been formally disseminated by the US Department of Agriculture and should not be construed to represent any agency determination or policy.”


